Polysilanes and Polycarbosilanes
PROPERTIES
Polysilanes are a class of heat stable inorganic polymers with a polymer backbone made up entirely of silicon-silcon bonds with two substituents attached to each silicon atom. The pendent groups are typically hydrogen, alkyl, and/or aryl groups. Thus an almost unlimited number of different polymers belong to this class. Their properties depend greatly on the nature of the side groups. Thus, these polymers cover a wide range of properties from highly crystalline and rigid to fully amorphous and very flexible solids. Most of these polymers have excellent heat resistance and are not very susceptible to hydrolysis but photodegrade when exposed to ultraviolet light.
Polysilanes possess unique electronic properties resulting from the interaction between the relatively large adjacent silicon orbitals which causes delocalization of sigma electrons in the Si-Si bonds, i.e. sigma-electron delocalization.1 These materials could function as photoresists and as photoconductors in microelectronic devices.
Until recently, polysilanes have found only a few applications because these polymers are difficult to produce and to process and are often of low quality and purity. However, recently, a new more practical method for the synthesis of polysilanes has been suggested.2 This method uses dichlorosilanes dissolved in a suitable solvent such as THF which is then polymerized via condensation reaction in the presence of Mg metal, LiCl and a Lewis acid such as ZnCl2 or FeCl2. This reaction has the advantage that it can be carried out at room temperature. However, it is only effective for the synthesis of polysilanes that have at least one aromatic substituent. Also, it has not been shown that this method is practical and feasible on an industrial scale.
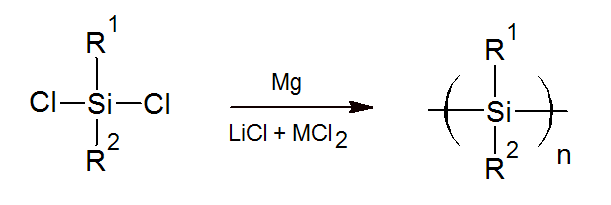
The alkyl groups along the chain can be substituted by many other groups (e.g., phenyl, vinyl, hydrogen, etc.).
Polysilanes are important precursors to silicon carbide fibers. A common method to produce these precursors is the Wurtz coupling method where an organohalosilane such as dimethyldichlorosilane is reacted with molten sodium metal to form polydialkylsilane:
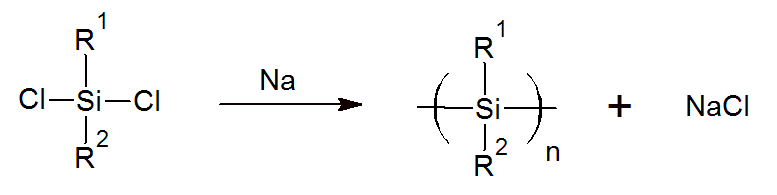
The precursor can be spun into fibers which are converted to polycarbosilane (SiC) fibers by pyrolysis in a subsequent step. These fibers have very high tensile strength.
Commercial Polysiloxanes
Polysilanes are rather expensive due to their low demand. Major producers of silane monomers and oligomers are Gelest, Wacker, and Dow Corning.
Applications
Polysilanes are mainly used as precursors for conversion to silicon carbide whereas the silane based polymers itself have found no or only a few commercial applications. The most important (potential) application appears to be in the microlithography for electronic devices. For example, high quality polysilanes could be employed as a hole transporting layer in organic light transmitting diodes (OLEDs) which are used for displays and other electronic devices. The polysilane based OLEDs could be particularly useful in flat panel displays.3 Polysilanes could be also part of the active layer of photo-voltaic elements to serve as the hole transporting layer.
Organofunctional silane monomers are sometimes incorporated into adhesives and sealants. They are either chemically bonded to the base polymer (STP = Silane Terminated Polymers), or they are added to formulated products for various purposes. For example, they function as crosslinkers in moisture curing adhesives and sealants or as adhesion promotors for a variety of substrates. They also improve the physical and mechanical properties of filler-reinforced materials.